New OS Data With Olaparib Support ‘New Era’ for Ovarian Cancer
May 14, 2020 10:00 amBy Liam Davenport
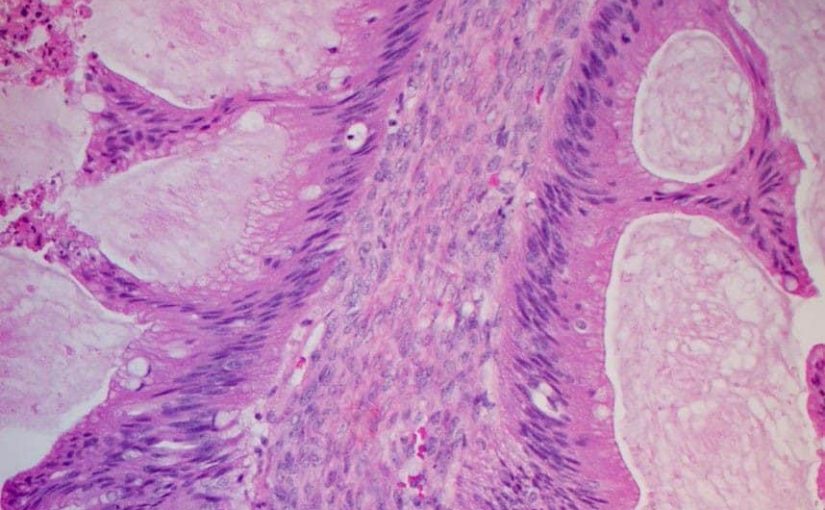
Women with platinum-sensitive relapsed ovarian cancer and a BRCA mutation could see their survival extended by over a year by maintenance therapy with the PARP-inihibitor olaparib (Lynparza, AstraZeneca).
The new overall survival (OS) data come … Read more