No Benefit to Adding Atezolizumab to Chemo/Bevacizumab for Ovarian Cancer
June 2, 2024 9:00 amby Silas Inman
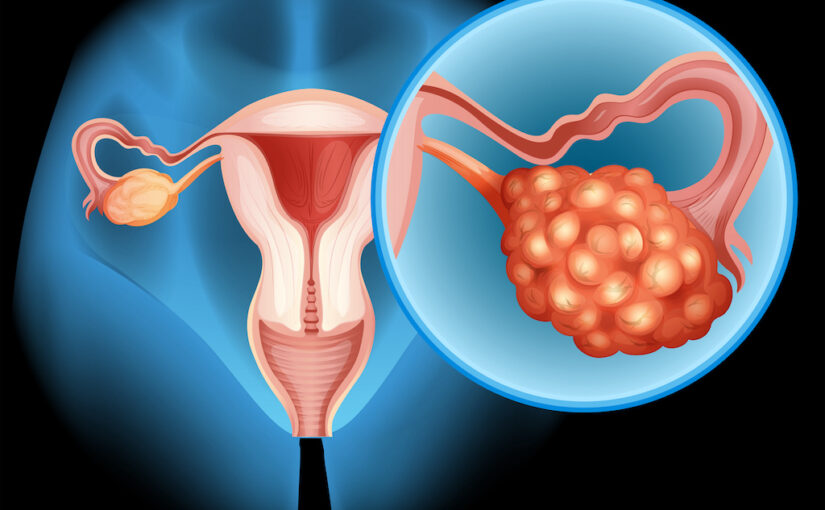
The addition of atezolizumab (Tecentriq) to single-agent non–platinum-based chemotherapy and bevacizumab (Avastin) did not provide any additional benefits for patients with recurrent ovarian cancer, according to the final analysis of the phase 3 AGO-OVAR 2.29/ENGOT-ov34 study (NCT03353831) … Read more