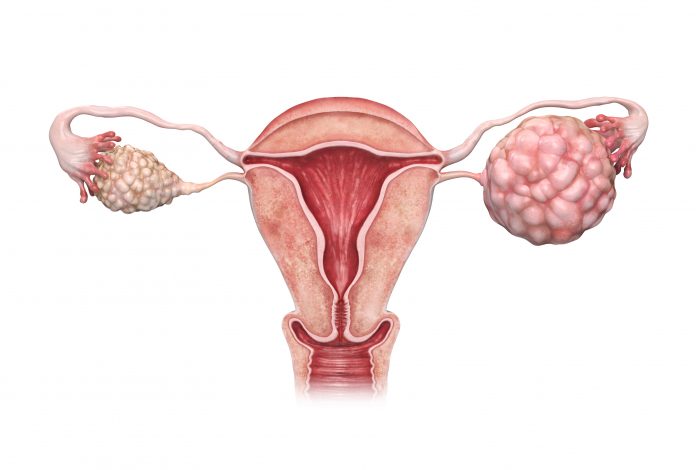
Ovarian Cancer: Best Practices for Managing PARP Inhibitor Side Effects
October 4, 2023 9:00 amOncologists treating ovarian cancer need to be proactive to prevent or reduce the likelihood of adverse effects associated with poly (ADP-ribose) polymerase (PARP) inhibitors, noted authors of a review in the ASCO Educational Book.
William Tew, MD, of Memorial Sloan … Read more