Individualized Dosing Regimen Reduces Side Effects in Ovarian Cancer Maintenance Therapy
March 18, 2019 7:00 pmBy Kristie L. Kahl
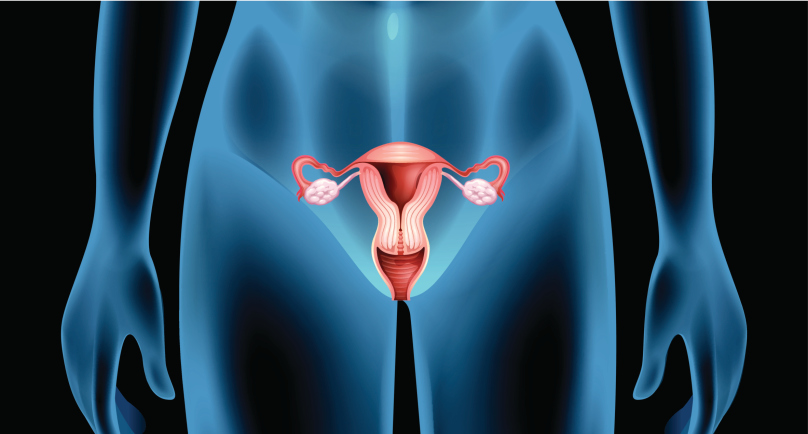
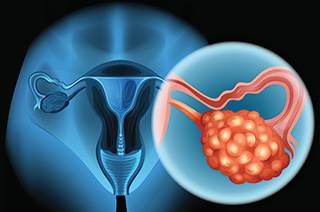
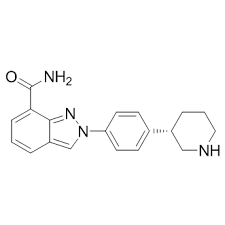
Side effects decreased among patients with high-risk ovarian cancer who received an individualized starting dose of Zejula (niraparib), based upon baseline bodyweight and platelet counts, compared with a fixed starting dose, according to a recent analysis
… Read more