STRO-002 Shows Encouraging Results in Phase I Trial in Treatment of Ovarian Cancer
April 27, 2020 10:15 amBy Peter Hofland, Ph.D
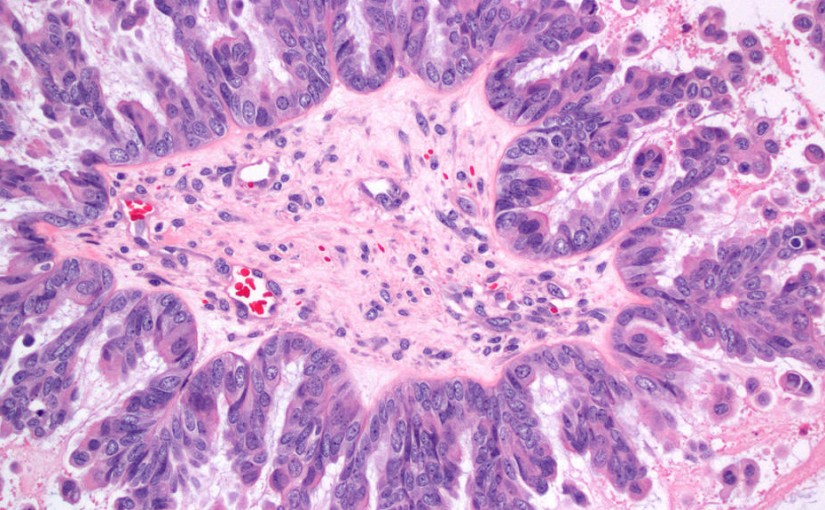
Updated interim data from an ongoing Phase I dose-escalation phase clinical study (NCT03748186) evaluating the folate receptor alpha (FRα) antibody-drug conjugate (ADC) STRO-002 (Sutro Biopharma) shows encouraging results.[1]
The trial was designed to study … Read more