A novel vaccine appeared safe and demonstrated clinical benefit as front-line maintenance therapy for women with advanced-stage ovarian cancer, according to results of a phase 2b trial presented during the virtual ASCO Annual Meeting.
Gemogenovatucel-T (Gradalis Inc.) showed benefit among women with BRCA wild-type and homologous recombination-proficient ovarian cancer.
“It is well-known that cancer immunology involves transmitting anti-immune signals to undergo an immune escape. Gemogenovatucel-T is an autologous tumor DNA immunotherapy derived from patients’ cancer cells at the time of surgery,” Rodney P. Rocconi, MD, chief of the gynecologic oncology service, associate director for clinical research and professor of interdisciplinary clinical oncology at University of South Alabama Mitchell Cancer Institute, said during his presentation. “Transfected with a plasma-encoded granulocyte-macrophage colony-stimulating factor in a bi-shRNA-furin inhibitor, gemogenovatucel-T was designed to enhance the immune system’s potency against cancer in three ways — it introduces the individual tumor-specific neoantigen repertoire to the immune system, enhances MHC presentation in CD8-positive T-cell activation via colony-stimulating factors, and it inhibits cancer expressive of TGF-beta with the bi-shRNA-furin, thereby blocking immunosuppressive cytokines. This collectively results in production of a cancer neoantigen-specific immune response that is patient-specific, where each vaccine is only good for a specific patient’s cancer.”
For the double-blind, placebo-controlled VITAL trial, researchers enrolled 91 women with newly diagnosed advanced-stage ovarian cancer who had a complete clinical response to front-line surgery and chemotherapy.
Researchers assigned women to gemogenovatucel-T (n = 47; median age, 63 years; 80.9% stage III disease) at a dose of 1 x 107 cells/mL intradermally or placebo (n = 44; median age, 62.5 years; 88.6% stage III disease) once monthly for up to 12 doses or until disease progression.
They used the Myriad myChoice CDx (Myriad Genetics) assay to determine women’s homologous recombination deficiency status, with scores of less than 42 deemed proficient.
RFS assessed by blinded independent central review served as the primary endpoint.
Among 62 women with BRCA wild-type disease who underwent homologous recombination deficiency testing, 45 were homologous recombination proficient.
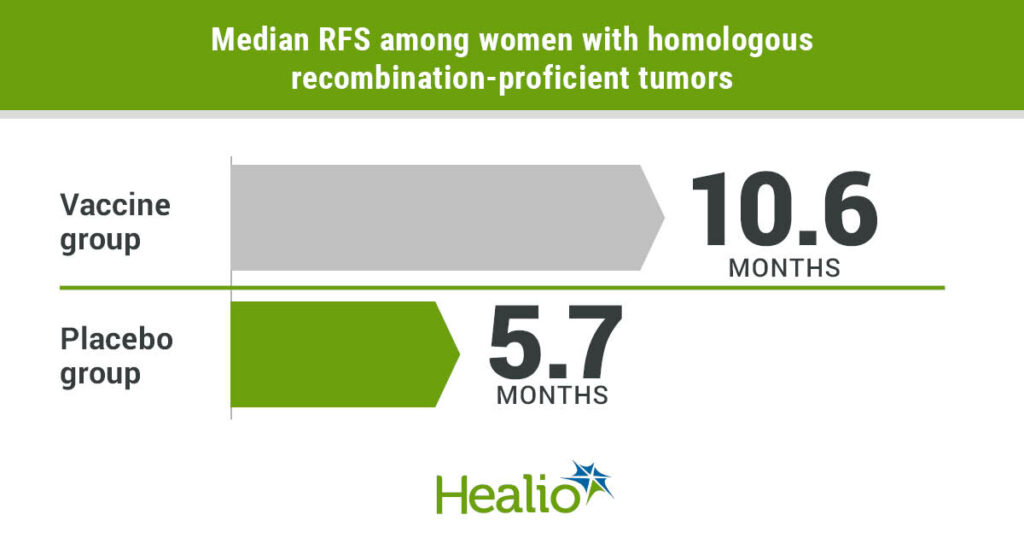
Results showed longer median RFS among women with homologous recombination-proficient tumors who were assigned to the vaccine group (n = 25) compared with those assigned placebo (n = 20; 10.6 months vs. 5.7 months; HR = 0.386; 90% CI, 0.199-0.75).

“However, RFS did not meet statistical significance for the overall cohort of patients and only showed a trend toward survival benefit,” Rocconi said.
Researchers also observed improved OS among women with homologous recombination-proficient tumors in the vaccine group vs. the placebo group (median, not reached vs. 26.9 months; HR = 0.342; 90% CI, 0.14-0.83).
“Restricted mean survival time has been validated as a beneficial methodology to calculate survival outcomes in immunotherapy, which estimates survival across entirety of time points that is similar to [area under the curve] calculation,” Rocconi said. “This approach validated our findings in the homologous recombination-proficient cohort, with statistically significant benefits in both RFS with a doubling from 10 months to 20 months, as well as an OS benefit from 28 months to 38 months with gemogenovatucel-T.”
At median follow-up of 58 months, researchers found the statistically significant OS benefit was maintained in the vaccine group, with an HR of 0.417 (P = .02). This translated into a significant improvement in 2-year OS (92% vs. 55%) and 3-year OS (70% vs. 40%) among women who received the vaccine.
“Overall, gemogenovatucel-T is a well-tolerated agent, with no grade 4 toxicities seen in the entire study. Most adverse events were injection site irritation, which [were] equal between the two groups and were mild in nature,” Rocconi said. “Without question, gemogenovatucel-T is a novel vaccine. In this trial, the vaccine demonstrated excellent tolerability that is ideal for maintenance therapy. Noteworthy was the enhanced efficacy in patients with homologous recombination-proficient tumors. Gemogenovatucel-T warrants further study.”
This article was published by Healio.


