by Josh Friedman
Key takeaways:
- AI-analyzed fragmentomes and protein biomarkers better distinguished women with ovarian cancer in screening.
- Liquid biopsy assay outperformed CA125 analysis alone.
An artificial intelligence-driven algorithm used a novel liquid biopsy assay to differentiate women with ovarian cancer from healthy patients, according to results presented at American Association for Cancer Research Annual Meeting.
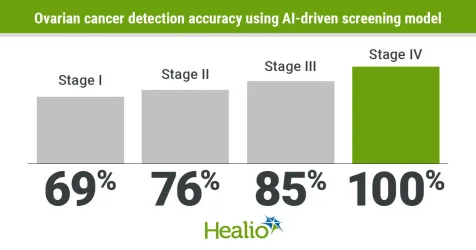
The analysis of cell-free DNA and specific protein biomarkers produced sensitivity rates ranging from 69% to 100% for stage I to stage IV ovarian cancer.
“We can detect ovarian cancer using an accessible, noninvasive artificial intelligence blood test with high sensitivity and specificity,” Victor Velculescu, MD, PhD, FAACR, co-director of the cancer genetics and epigenetics program at Sidney Kimmel Comprehensive Center at Johns Hopkins, told Healio.
“Our assay found the vast majority of ovarian cancers successfully while avoiding false-positive results,” he added. “This is a promising avenue for early detection of ovarian cancer and broad screening of this disease.”
Background and methodology
Ovarian cancer causes the most deaths from gynecologic cancer globally, serves as the fifth most common cause of cancer deaths in women in the U.S. and has a 5-year survival rate around 50%, according to background information provided by researchers and AACR.
“Ovarian cancer is a devastating disease with no widely used effective screening available,” Velculescu said. “The lack of efficient screening tools as well as the asymptomatic development of ovarian cancer contributes to late diagnoses when treatment options are limited. We felt it was important to develop a cost-effective, accessible detection method that could change clinical paradigms for ovarian cancer detection and save lives.”
Velculescu and colleagues had previous success with the DNA evaluation of fragments for early interception (DELFI) model in patients with lung cancer, which led to the launch of the FirstLook Lung clinical test in October 2023, “the first validated liquid biopsy test for lung cancer screening,” he said during his presentation.
Researchers attempted to replicate that success in ovarian cancer.
They used the DELFI method, dubbed DELFI-Pro for this study, which evaluated cell-free DNA fragmentomes and two different protein biomarkers — cancer antigen 125 (CA125) and human epididymis protein 4 (HE4) — using a trained machine learning algorithm.
“Cell-free DNA fragmentomes represent a genome-wide compendium of [cell-free] DNA fragments in the blood, providing an integrated view of the chromatin, genomic and transcriptomic states of healthy and cancer cells of an individual,” Velculescu said. “Because cancer cells are rapidly growing and dying, and have chaotic genomic organization as compared with healthy cells, individuals with cancer have different kinds of cfDNA fragments in their blood than patients without cancer. By carefully analyzing these DNA fragments across the entire human genome, we can detect subtle patterns of changes indicating the presence of cancer.”
The investigation compared discovery and validation cohorts from three sets of women — those with ovarian cancer (n = 134), those without cancer (n = 204) and those with benign adnexal masses (n = 203).
Results and next steps
The liquid biopsy assay had greater than 99% specificity for detecting stage I ovarian cancer. Researchers reported 72% sensitivity for detection of stage I ovarian cancer, 69% for stage II, 87% for stage III and 100% for stage IV [area under the curve = (AUC) 0.96; 95% CI, 0.94-0.99].
They also measured the specificity for CA125 alone and observe rates of 34% for stage I, 62% for stage II, 63% for stage III and 100% for detection of stage IV ovarian cancer.
The algorithm identified 91% of high-grade serous ovarian cancers with greater than 99% specificity.
“This method is robust for detecting ovarian cancer and is generalizable across different populations,” Velculescu said.
The AI-driven algorithm also separated benign masses from ovarian cancers at a high level (AUC = 0.87; 95% CI, 0.83-0.91).
“Our estimates are that the positive predictive value of DELFI-Pro is 16% while those of CA125 are 5% or lower, and given the low prevalence of ovarian cancer and the risks of exploratory surgery, a PPV greater than 10% is needed to justify population-wide screening for ovarian cancer in a way that balances the benefits of early detection against the potential harm from unnecessary surgical procedures,” Velculescu said during his presentation.
The retrospective nature of the study and the small sample size limit the applicability of the results, according to researchers. They plan to validate their results in larger study cohorts.
“In addition to screening, there is the diagnostic value of our approach in helping clinicians distinguish between benign and malignant ovarian masses, and we will work in larger studies to validate our method in this setting as well,” Velculescu said.
References:
- AACR. Artificial intelligence analysis of DNA fragmentomes and protein biomarkers noninvasively detects ovarian cancer (press release). Available at: https://www.aacr.org/about-the-aacr/newsroom/news-releases/artificial-intelligence-analysis-of-dna-fragmentomes-and-protein-biomarkers-noninvasively-detects-ovarian-cancer/. Posted April 9, 2024. Accessed April 9, 2024.
- Medina JE, et al. Abstract 6086. Presented at: American Association for Cancer Research Annual Meeting; April 5-10, 2024; San Diego.
Source: Medina JE, et al. Abstract 6086. Presented at: American Association for Cancer Research Annual Meeting; April 5-10, 2024; San Diego.
Disclosures: Delfi Diagnostics funded the study. Velculescu reports being the founder and member of the board for Delfi Diagnostics, founder of Personal Genome Diagnostics and member of the scientific advisory board at Epitope and Viron Therapeutics. Velculescu is an inventor on patent applications related to cancer genomic analysis and cell-free DNA for cancer detection that have been submitted by Johns Hopkins University and licensed to Agios, Delfi Diagnostics, Esoterix, Genzyme, LabCorp, ManaT Bio, Qiagen, Sysmex and Ventana. Please see the abstract for all other researchers’ relevant financial disclosures.
Published by: Helio

