Biomarker Selection and Results Interpretation
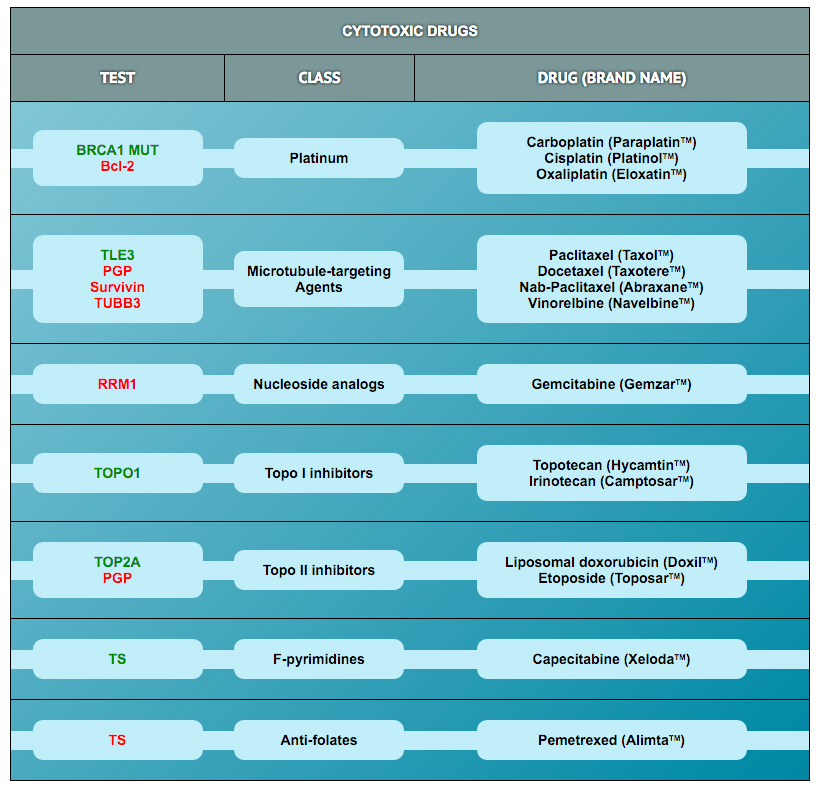
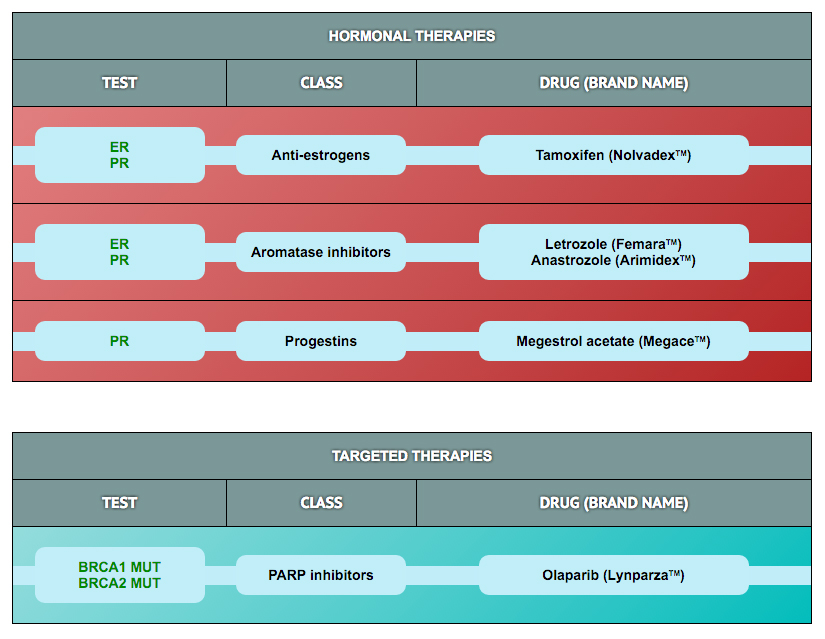
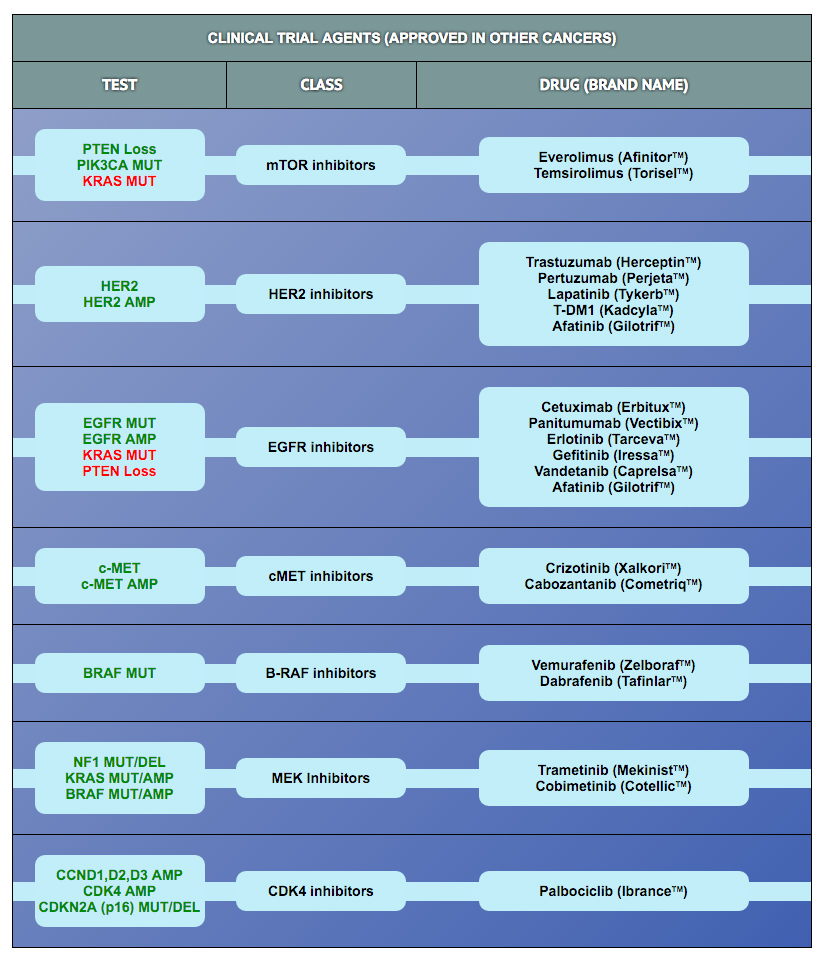
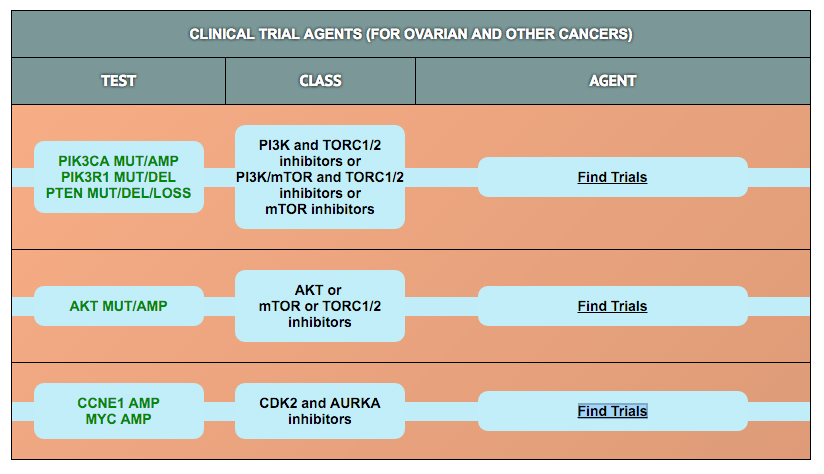
The protein biomarker tests in the panel were selected based on results published in retrospective clinical research studies in ovarian and other cancers. Those studies demonstrated an association of biomarker expression levels with response to drug treatment (i.e., with sensitivity or resistance). Clearity has defined cut-points for high and low expression for most of these markers based on data derived from >500 patient specimens included in the Clearity database.
In addition to the protein tests, the analysis also looks for mutations or other genomic alterations (e.g., copy number, fusions) in 300 genes known to be involved in cancer etiology or progression. Importantly, some alterations in these genes have been associated with responses to drugs that are currently being tested in clinical trials (some have already been approved to treat patients with other cancer types). The drug response correlations for the most frequently identified genetic alterations in tumors from ovarian cancer patients are provided.
To learn more about the mechanism of action of each drug and how the biomarker is associated with drug activity, please click on the drug of interest in the tables.